- * 프린트는 Chrome에 최적화 되어있습니다. print
DNA is the building block for the genome and provides the blueprint for almost every aspect of life. DNA can be damaged by endogenous assaults such as replication errors, oxidative stress or by exogenous challenges such as radiation or toxic chemicals. Failure to repair such damage results in cell death or the accumulation of changes in DNA that can cause genetic diseases including cancers or can lead to premature aging.
Research at the IBS Center for Genomic Integrity is concerned with the many DNA damage response and repair pathways that can sense and repair damaged DNA and signal to cells that there is an urgent problem to be addressed. We also study the link of these pathways to other parts of cellular metabolism, including DNA replication, cell cycle regulation or transcription.
Our research spans many disciplines and techniques, including molecular, cell, chemical and structural biology, genetics, biochemistry and the use of animal models. Finally, we are interested in leveraging our research to develop small molecule modulators of DNA repair pathways as probes and to exploit vulnerabilities of tumors in therapeutic settings.
Major research field
Synthetic lethality, DNA Repair, DNA Recombination, DNA Damage Response, Cancer, Aging
Desired field of research
DNA Repair, Synthetic lethality, Cancer, DNA Damage Response, ATAD5, PCNA, CRISPR, CRISPR-Cas9
Research Keywords and Topics
Composition For Inducing Apoptosis of Cancer Cell And Method For Inducing Apoptosis of Cancer Cell Using the Same
Molecular Analysis of DNA replication, repair, recombination genes and proteins
Cellular Response to DNA damaging agents
Animal models to understand defects in DNA replication, repair, recombination
Identification of potential precision therapeutic molecules for cancers and aging
Research Publications
MORE- Kwon T#, Ra JS, Lee S, Baek IJ, Khim KW, Lee EA, Song EK, Otarbayev D, Jung W, Park YH, Wie M, Bae J, Cheng H, Park JH, Kim N, Seo Y, Yoon S, Kim HE, Moon HE, Paek SH, Park TJ, Park YU, Lee H, Choi JH, Cho SW#, Myung K#. (2022) Precision cell death of tumors by targeting InDel mutations by CRISPR-Cas9. Proc. Natl. Acad. Sci. USA. #, Co-correspondence. In press
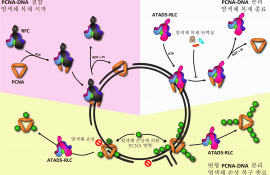
- Park SH, Kang N, Wie M, Lee D, Lee EA, Hwang SH, Ra JS, Park IB, Kim N, Park JH, Lee KY#, Myung K#. (2019) ATAD5 promotes replication restart by regulating RAD51 and PCNA in response to replication stress. Nature Communication 10:5718. #,Co-correspondence
- Kang MS, Ryu E, Lee SW, Park J, Ha NY, Ra JS, Kim YJ, Abdel-Rahman M, Kim H#, Kang S#, Myung K# (2019) Regulation of PCNA cycling on replicating DNA by RFC and RFC-like complexes. Nature Communication 10:2420 #,Co-correspondence
Patents
U.S. Patent No. 61/930,291 (E-039-2014/0-US-01) Compounds and Method for treating PARP1-deficient cancers
Korean Patent No. P2018-0059-P1-D1.Composition for Inducing Apoptosis of Cells With Modified Genes
Korean Patent No. (10-2019-0157333) Development of synthetic Baicalein derivatives as a chemotherapeutic agent
Korean Patent No. (10-2020-0081538) 유전자가 변이된 세포의 사멸 유도 조성물 및 상기 조성물을 이용한 유전자가 변형된 세포 사멸 유도 방법
국가과학기술표준분류
- LA. 생명과학
- LA01. 분자세포생물학
- LA0199. 달리 분류되지 않는 분자세포 생물학
국가기술지도분류
- 건강한 생명사회 지향
- 021600. 유전자조작/전달기술
녹색기술분류
- 녹색기술관련 과제 아님
- 녹색기술관련 과제 아님
- 999. 녹색기술 관련과제 아님
6T분류
- BT 분야
- 기초/기반기술
- 020114. 생명현상 및 기능연구




